Vaccine Trial Centre: HPV Vaccine Project Timeline
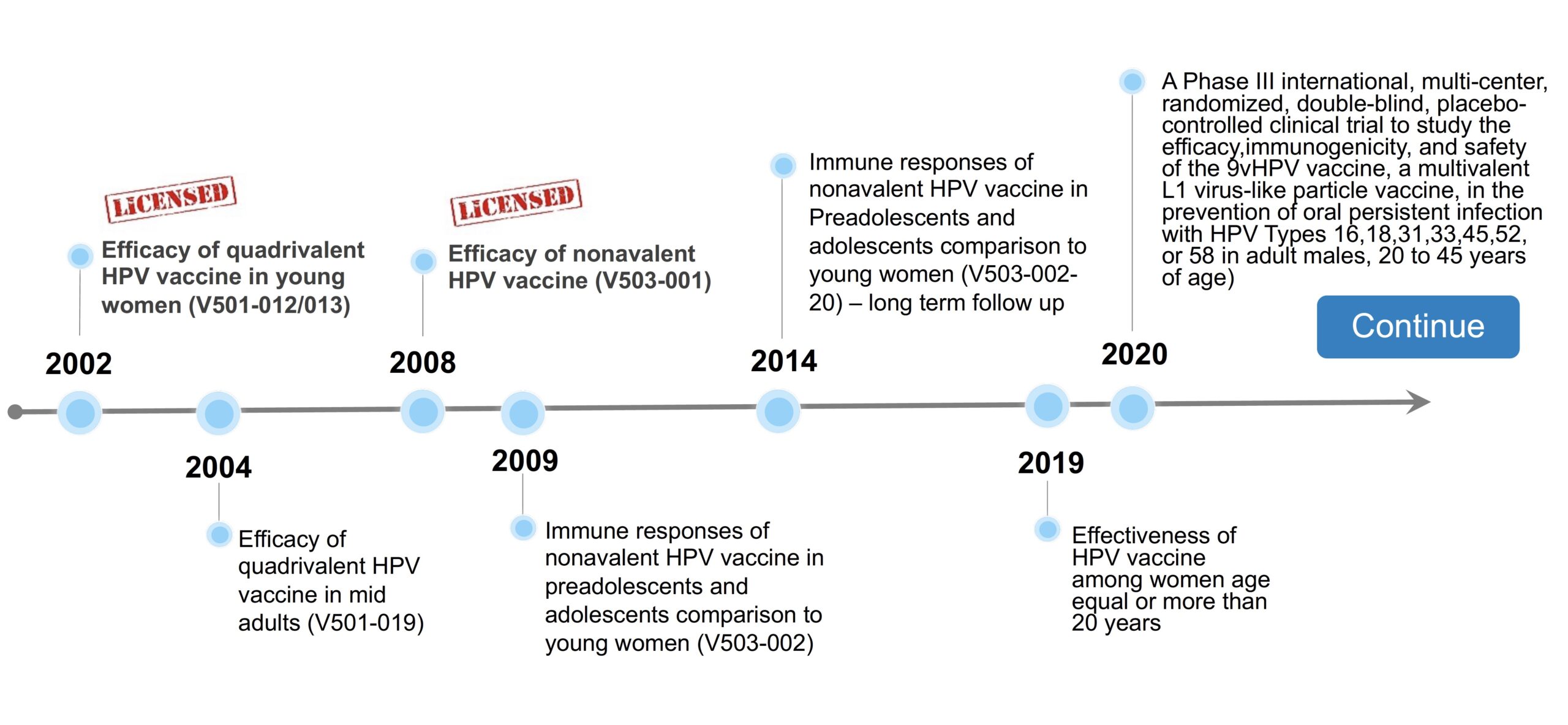
2002
Efficacy of quadrivalent HPV vaccine in young women (V501-012/013) 𝗟𝗶𝗰𝗲𝗻𝘀𝗲𝗱
2004
Efficacy of quadrivalent HPV vaccine in mid adults (V501-019)
2008
Efficacy of nonavalent HPV vaccine (V503-001) 𝗟𝗶𝗰𝗲𝗻𝘀𝗲𝗱
2009
Immune responses of nonavalent HPV vaccine in preadolescents and adolescents comparison to young women (V503-002)
2014
Immune responses of nonavalent HPV vaccine in Preadolescents and adolescents comparison to young women (V503-002-20) – long term follow up
2019
Effectiveness of HPV vaccine among women age equal or more than 20 years
2020
A Phase III international, multi-center, randomized, double-blind, placebo-controlled clinical trial to study the efficacy, immunogenicity, and safety of the 9vHPV vaccine, a multivalent L1 virus-like particle vaccine, in the prevention of oral persistent infection with HPV Types 16,18,31,33,45,52, or 58 in adult males, 20 to 45 years of age)

